An amniotic membrane is part of the fetal placenta. It is the tissue closest to the baby throughout development in the womb. Amniotic membrane protects the baby from any harm and it has natural therapeutic actions which help the baby develop. The placentas used to prepare amniotic membranes are donated by consenting mothers after cesarean section (C-Section) births. Mothers that donate are fully informed, have healthy lifestyles, and are tested against infectious diseases prior to donation. Stem cells from these membranes are used in a variety of medical procedures, including in amniotic membrane grafts.


Amniotic membranes are rich with fetal stem cells and can function in the eye as a basement membrane substitute or as a temporary graft. It has anti-inflammatory and anti-scarring effects and contains growth factors that promote wound healing on the surface of the eye. The fetal stem cells interact with our cornea’s limbal stem cells to enhance their health and promote proper function to enhance healing. Amniotic membrane therapy has been found to be a good alternative for corneal and conjunctival reconstruction in many clinical situations.

There are many indications for usage of an amniotic membrane.
These include (but are not limited to):
You may need either a sutured amniotic membrane graft or a PROKERA® sutureless amniotic membrane depending on the reason for treatment and the severity of your ocular disease. A sutured amniotic membrane may be used in more serious cases, or when multiple layers and stability of the graft are important and is done in the Ambulatory Surgery Center.
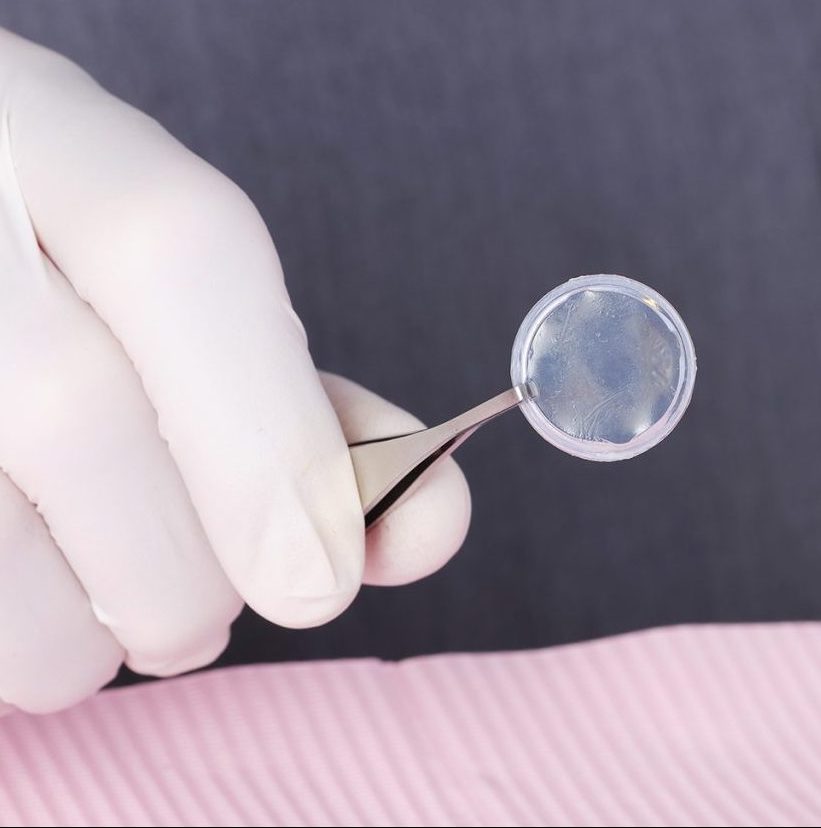
PROKERA® is a medical device composed of an amniotic membrane mounted within a ring. Think of PROKERA® as a “living contact lens” that is placed on the surface of your eye. Similar to a sutured amniotic graft, PROKERA® provides patients a treatment option for ocular surface and corneal wound healing due to its anti-inflammatory and anti-scarring properties. The PROKERA® procedure is done in the office minor treatment room.